Key Clinical Advances: New Epinephrine Approval and Seizure Treatment Data
Introduction
Clinical research continues to deliver important therapeutic progress across emergency care and neurologic disorders.
Recent updates from Clinical Trial Vanguard highlight a new FDA approval for hospital-use epinephrine and emerging long-term seizure treatment data with promising clinical responses.
Below is a summary of three noteworthy developments impacting patient care and treatment paradigms.
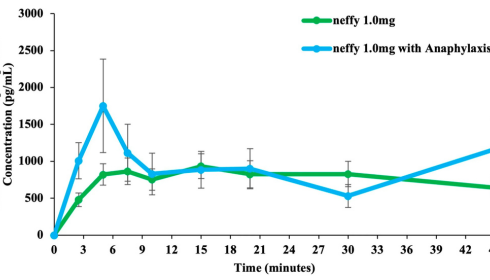
1. FDA Approves Amneal’s New Epinephrine for U.S. Hospitals
The FDA has approved a new epinephrine product developed by Amneal for use in U.S. hospitals.
🔗 Read more: FDA Approved Amneal’s New Epinephrine for U.S. Hospitals
Epinephrine is a critical lifesaving medication used in acute emergency settings, including cardiac arrest and anaphylaxis. The new approval expands hospital options for this essential drug, which may contribute to enhanced supply redundancy and further support emergency care providers.
This regulatory milestone reinforces ongoing efforts to ensure reliable access to first-line emergency medications in clinical settings where rapid response can be life saving.
2. Promising 48-Month Azetukalner Epilepsy Study Data by Xenon
In the field of epilepsy research, Xenon Pharmaceuticals has published encouraging 48-month clinical data for its investigational therapy azetukalner.
🔗 Read more: Promising 48-Month Azetukalner Epilepsy Study Data by Xenon
Long-term data from epilepsy studies are essential to understanding the sustained impact of therapeutic candidates on seizure frequency and patient quality of life. The 48-month findings indicate that azetukalner continues to demonstrate favorable safety and effectiveness profiles over extended follow-up, offering hope for patients with challenging seizure disorders.
These results reinforce the potential role of azetukalner as a valuable treatment option in the long-term management of epilepsy.
3. RAP-219’s Consistent Clinical Response in Focal Seizures
Another notable development in seizure therapeutics involves RAP-219, which has shown consistent clinical responses in patients with focal epileptic seizures.
🔗 Read more: RAP-219’s Consistent Clinical Response in Focal Seizures
Focal seizures, which originate from specific regions of the brain, can be especially disruptive and difficult to manage. Consistent response patterns in clinical assessment suggest that RAP-219 may provide meaningful benefit for patients whose seizures are resistant to current therapies.
Continued evaluation in larger patient populations will help define RAP-219’s role in future epilepsy treatment landscapes.
Conclusion
From a new emergency care medication approval to long-term epilepsy research data, these clinical trial developments reflect the dynamic progress occurring across acute and chronic neurologic conditions.
For more detailed coverage of trials and emerging therapeutic insights, visit Clinical Trial Vanguard.
SEO Meta Information
Meta Title:
Clinical Trial Highlights: New Epinephrine Approval and Epilepsy Treatment Advances
Meta Description:
Explore the latest clinical updates including FDA approval of new hospital-use epinephrine, Xenon’s 48-month azetukalner epilepsy study data, and RAP-219’s consistent response in focal seizures.
Keywords:
FDA epinephrine approval, Amneal epinephrine, azetukalner epilepsy data, RAP-219 focal seizures, clinical trials, neurological research, Clinical Trial Vanguard